-
Raleigh Local Delivery
-
Organically Grown
-
Raleigh Local Delivery
-
Organically Grown
-
Raleigh Local Delivery
-
Organically Grown
-
Raleigh Local Delivery
-
Organically Grown
-
Raleigh Local Delivery
-
Organically Grown
-
Raleigh Local Delivery
-
Organically Grown
Rooted in Nature. Wild at Heart.
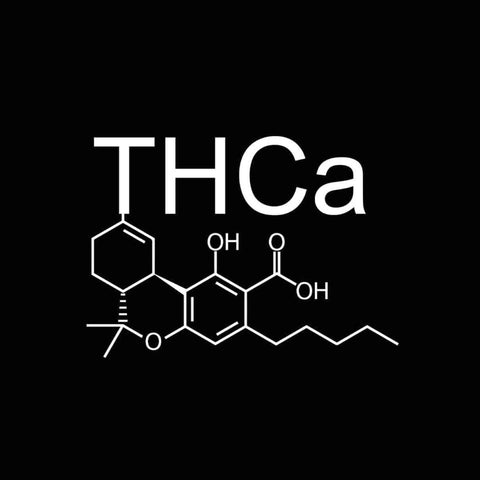
At Wildflower Hemp Co, we grow premium THCa hemp flower in living soil, cure it slow, and ship it fast. We’re a small, owner-run crew with a passion for the plant and a love for the wild ride this industry brings.
Crafted with experience. Delivered with care.
100% federally legal thc. No med card required.
-
Lab Tested
-
Worldwide
-
Discreet Shipping
-
Lab Tested
-
Worldwide
-
Discreet Shipping
-
Lab Tested
-
Worldwide
-
Discreet Shipping
-
Lab Tested
-
Worldwide
-
Discreet Shipping
-
Lab Tested
-
Worldwide
-
Discreet Shipping
-
Lab Tested
-
Worldwide
-
Discreet Shipping